Publications IPH Magazine Revista IPH Nº 15 Implementing an Integrated System for Clinical Engineering Management
Implementing an Integrated System for Clinical Engineering Management
Alessandro de S. Mota, Cleiton V. Caldeira, Dennis V. do Nascimento, Diego H. L. da Silva Ribas, Fabio Martins Correa, Ferdinando Silvestre de Melo, Francisco Claudio Cardoso Gomes Benetti, Gustavo Borjes Sanjinez, Ilan A. Kimura, Katia K. Hara

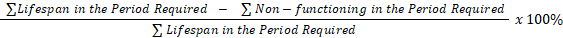
1. Introduction and the problem ahead of us
The lack of an integrated management system made it difficult to inventory medical and hospital equipment; get the accurate history of equipment; carry out preventive maintenance programs, calibration and electrical safety test; which are means to ask the clinical engineering staff for opening service requests that enable monitoring, the development of performance and cost guidelines, the control of contract maintenance and tracking maintenance and actions performed.
Knowing the quantity and quality of existing medical and hospital equipment is key to structure a clinical engineering service. Although obtaining the data for inventory is rather a simple task, in this case, the great difficulty was the complexity and size of HC-FMUSP complex, which is comprised of ten institutions: eight institutes and two auxiliary hospitals that adds up to approximately 2,400 (two thousand and four hundred) beds, 80 (eighty) operating rooms and 600,000 (six hundred thousand) square meters of built area, from which the project contemplates five institutes and one auxiliary hospital.
2. Background and the proposed solution
Illustration 1, bellow, portraits in brief the implementation of the system background:

Illustration 1 - Implementation of the System Background
Acquisition: In this phase, we carried out a study regarding existing systems in the market that dealt with analysis of functionalities, the acquisition, and the parameterization of the basic criteria of the system, which initially consisted of the development of the descriptions of the equipment families, followed by the parameterization of important points, such as occurrences, causes and registration of work orders, that is, the main defects presented by the equipment, the reasons that cause an equipment to present a defect and the activities performed by the technical team. We also defined he types of maintenance were also defined, among them, installation, corrective, preventive, calibration, electrical safety test, user support, etc.
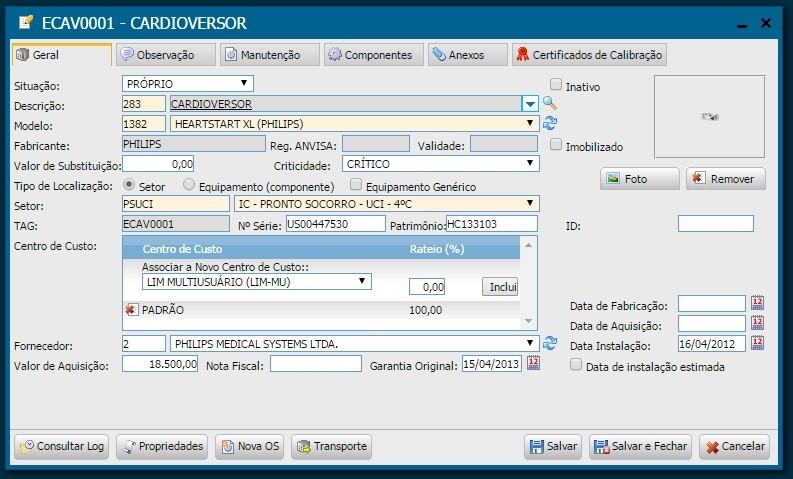
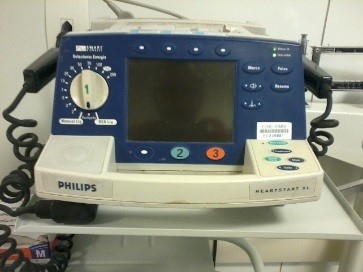
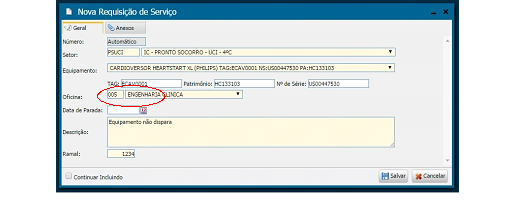
Implementation/Merger: In this phase, we inventory the equipment based on physical data, (serial number, patrimony, brand, model, etc.) by performing on-site gathering within healthcare facilities, subsequent insertion in the management system. For this task, it was also necessary to register manufacturers and suppliers; models and criticalities of the equipment; and the auxiliary departments to finally proceed with the registration of the equipment. At the end of each registration, a TAG, automatically generated by the system, is printed and affixed to the equipment. Illustrations 2 and 3 bellow show the equipment registration screen and s sample registered cardioverter.
Since the Children's Institute (ICR) used the system in an individual and isolated manner, the implementation phase was characterized by the migration of the database to the new integrated base in accordance with parameters defined in the new management model.

Illustration 2 - Equipment Registration Screen

Illustration 3 - Cardioverter - ECAV0001
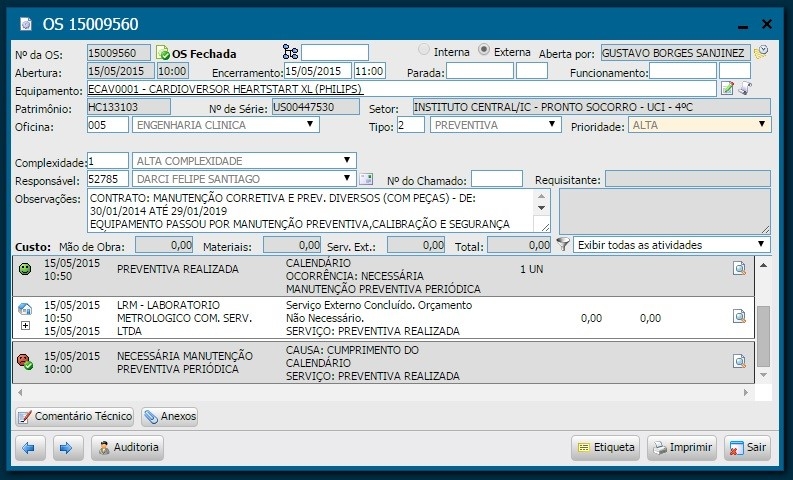
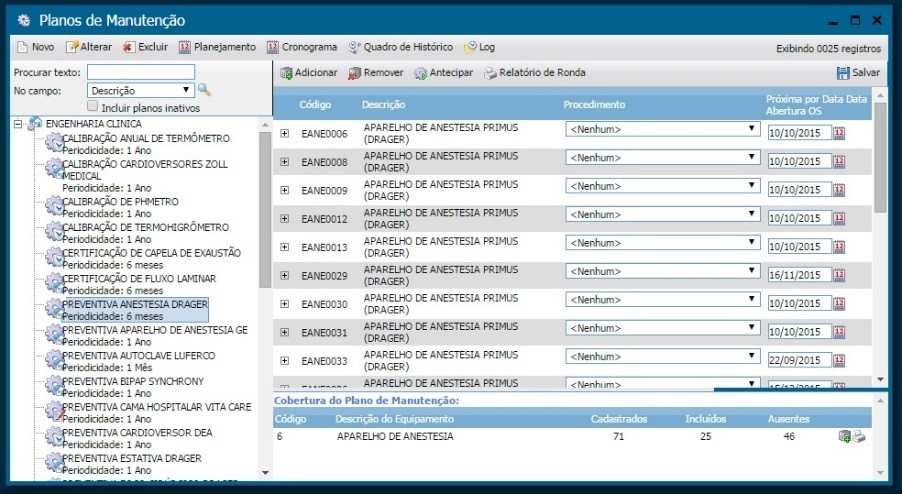
During the implementation phase, we carried out training sections with the clinical engineering team to try other system tools, such as the work orders module, maintenance plans, contracts, etc. Illustrations 4, 5 and 6 present the screens regarding the aforementioned tools.

Illustration 4 - Work order screen

Illustration 5 - Maintenance Plan Screen

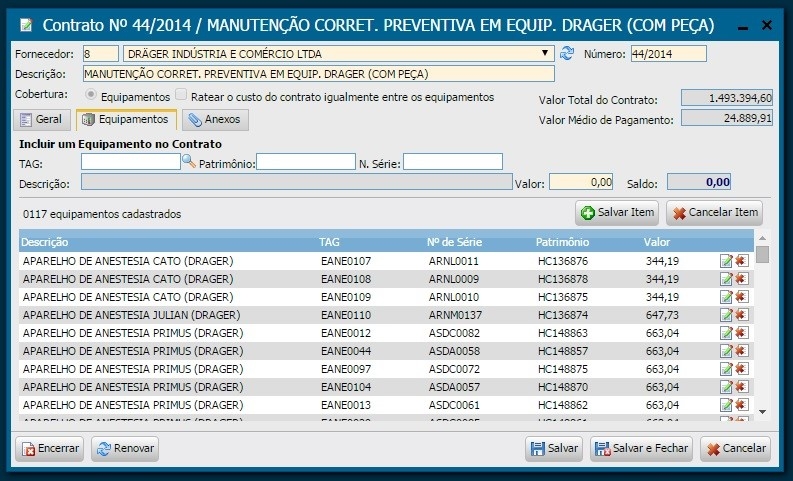
Illustration 6 - Contract Screen
Besides training with the clinical engineering team, the healthcare team also learned how to use the system, since it allows all HC staff to request a service, proceed with follow-up and even evaluate the service provided.
3. Results
After implementing the maintenance management system, we reached the following outcome:
3.1. Inventory
As a result of implementing the project within the aforementioned institutions, we registered about 17,000 equipment.
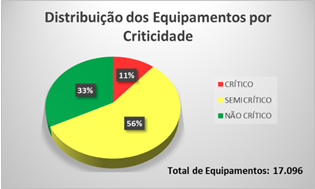
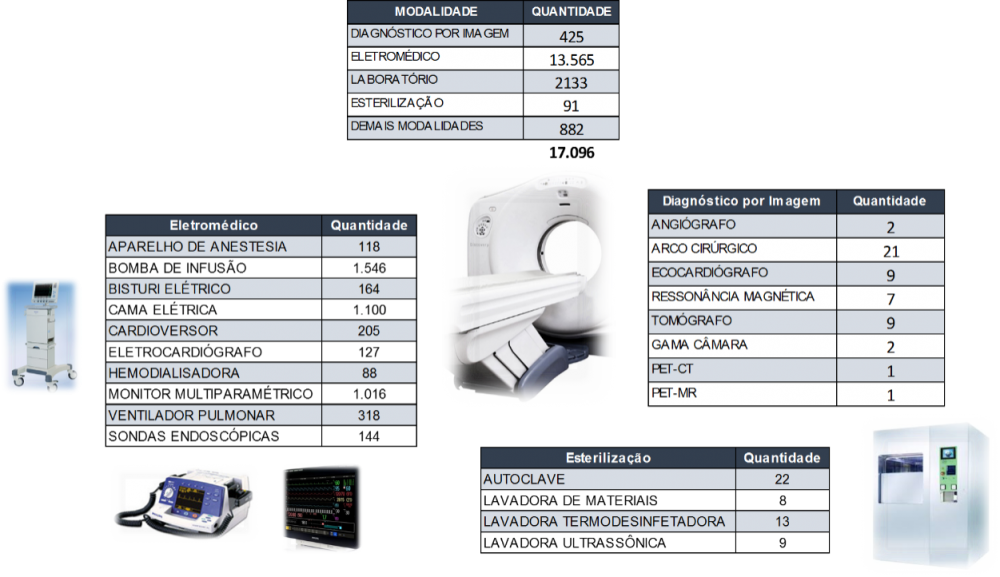
Illustrations 7 and 8, show the distribution of equipment sorted by criticality and some specialty.
Once we had the criticalities defined, it was possible to implement the work order priorities.

Illustration 7 - Equipment sorted by Criticality

Illustration 8 - Equipment sorted by Specialties
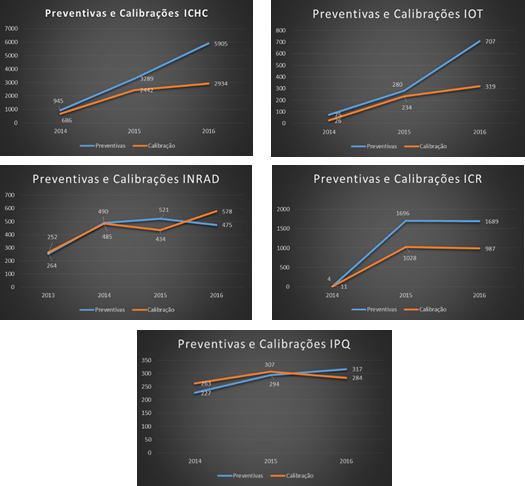
3.2. Maintenance Planning
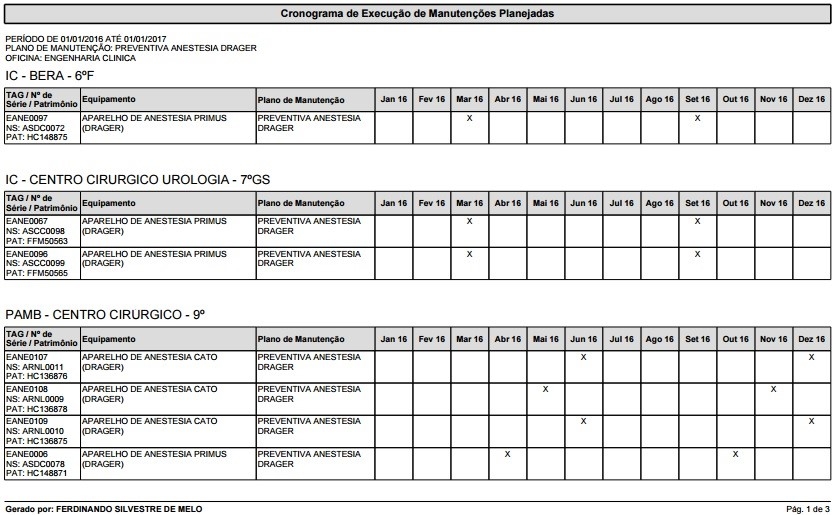
Once we had carried out the inventory, it was possible to perform a thorough analysis of existing equipment and implement a maintenance program based on priorities using the records of maintenance plans, automating the process of opening work orders, improving control and enabling the automatic schedule of maintenance procedures. Illustrations 9 and 10 show a sample timetable generated by the anesthesia device plan for the year 2016 and the list of maintenance procedures scheduled by each institution.

Illustration 9 - Preventive Maintenance Schedule Automatically Generated by the System

Illustration 10 - List of Scheduled Maintenance Procedures by Institution
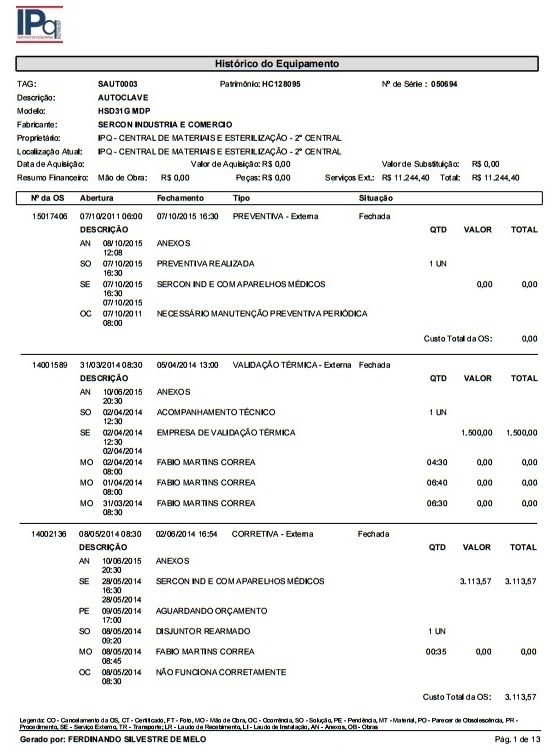
3.3. Background of the Equipment
By referring to the equipment background performance data, it is possible to analyze its life cycle and make decisions regarding the need for maintenance or technology replacement.
Illustration 11 portraits the maintenance background of an autoclave installed at the Institute of Psychiatry, including the work orders and costs.

Illustration 11 - Equipment Maintenance Background
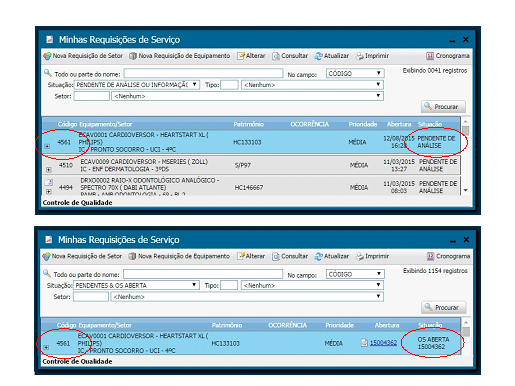
3.4. Opening and Following up a Client Work Order
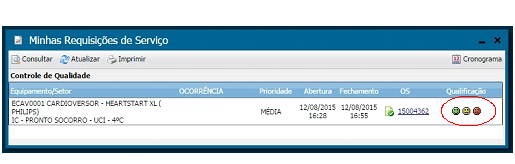
With the new system, all employees from the aforementioned institutes may request a service, proceed with the follow-up and even assess the service provided, the latter being mandatory, since it is not permitted to open a new requisition before evaluating a previously completed one. We keep track of maintenance during its procedure and after its completion to improve the workflow and ensure bidirectional communication and traceability of actions and information. Moreover, the system saves paper by avoiding printing.
Illustrations 12, 13 and 14 bellow present the screens regarding the opening, follow-up and assessment of work orders.

Illustration 12 - Opening a Work Order Screen

Illustration 13 - Work Order Follow-up Screen

Illustration 14 - Work Order Assessment Screen
3.5. Indexes
By implementing the system, we were able to standardize indexes for the management of the technology park. Besides analyzing the performance of the service provided, we are able to accomplish a more detailed study of the performance of the equipment, as well as to compare results among the institutes. Currently, we are using the following indexes: Respect for the Preventive Maintenance Schedule; Equipment Availability; General Maintenance Cost; Technical Team Productivity; Causes for the Work Orders; Clinical Engineering Response Time; Pending Work Orders, among others.
The illustrations bellow show some of those indexes.

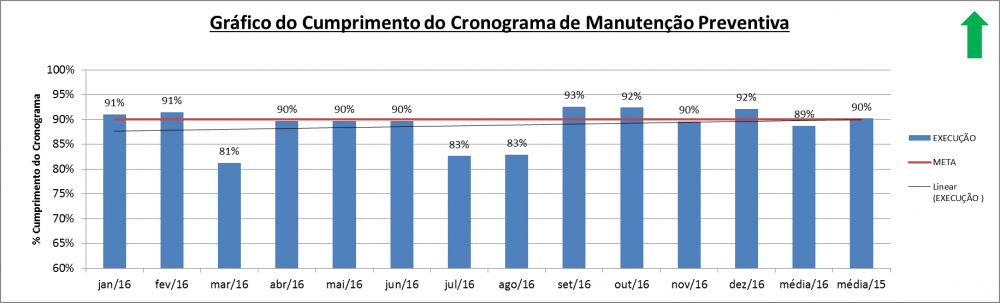
Illustration 15 - Respect for the Preventive Maintenance Schedule Index
Description: Percentage of execution of the preventive maintenance scheduled in a month
Calculation: (∑ Preventive Maintenance Carried Out / ∑ Preventive Maintenance Scheduled) x100%

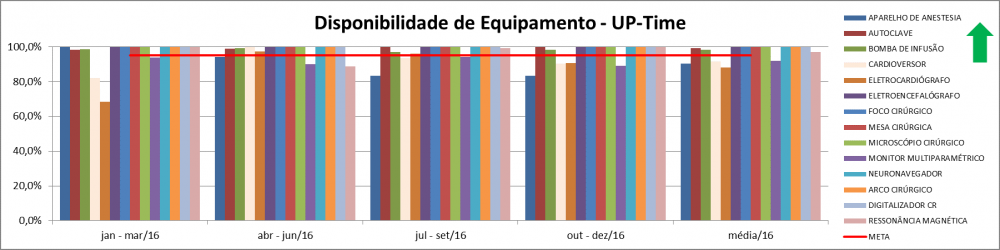
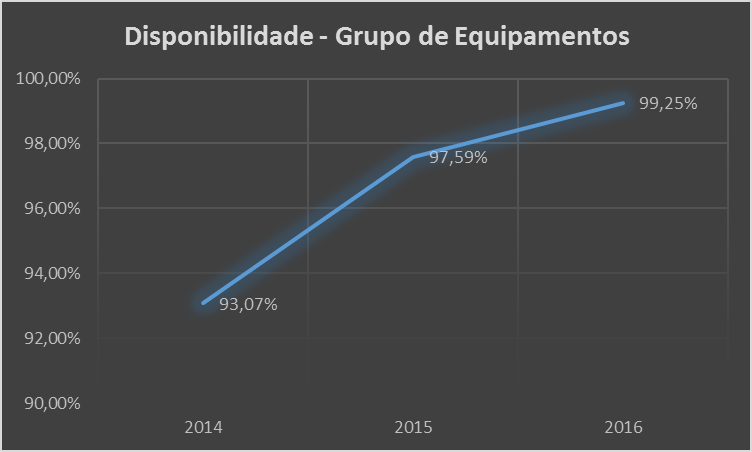
Illustration 16 - Equipment Availability Index
Description: Percentage of time the equipment was available for use
Calculation:
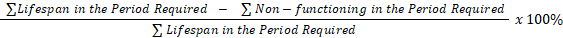

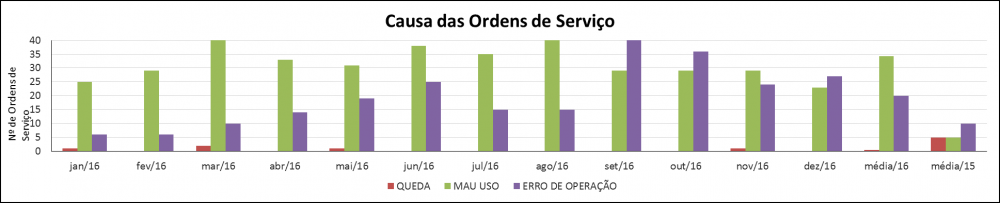
Illustration 17 - Causes for the Work Order Index
Description: What triggered the work order
Calculation: ∑ Total Amount of Causes by Sort
3.6. Contracts
The implementation of the system provided greater visibility of existing contracts, which, in turn, enabled improvements, inclusion of new equipment and, above all, the unification of several contracts from the same manufacturer into a single one.
Bellow, we list some benefits from unifying contracts:
- Greater control and better management of the contract and less administrative processes;
- Reduction and unification of costs for the same equipment by comparing prices and taking as reference the lowest price among the contracts to be unified;
- For contracts where it was not possible to reach a better value, we improved the technology gain by, for instance, incluing preventive kits in contracts for pulmonary ventilators, transducers in ultrasound contracts, etc.;
- In addition to the inclusion of corrective and preventive maintenance parts, for some contracts, we also included consumables / accessories to reduce equipment's downtime - whether due to maintenance problems or lack of an accessory - to the fullest, hence increasing its availability to the user;
- Decrease of single and distinctive processes to purchase parts and / or services;
- Increase of equipment availability.
The benefits mentioned above, lead to several additional qualitative benefits, such as: increasing high-quality exams, enhanced safety for patients and users of equipment, etc.
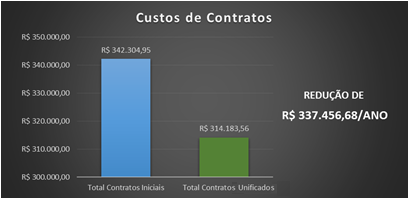
Illustration 18 presents the advantage in HC-FMUSP's annual economic result accomplished after unifying the contract of a supplier of diagnostic imaging equipment with an increase in technology. Illustration 19 shows improved performance regarding Institute of Radiology's equipment belonging to the aforementioned contract.

Illustration 18 - Economic Impact for HC-FMUSP after the Unification of Contracts

Illustration 19 - Performance Outcome of INRAD Equipment after the Unification of Contracts
3.7. Hospital Accreditation
Considering the topics mentioned above, it is safe to say the implementation of the system, i.e. the clinical engineering department, was elemental for the success of the hospital accreditation process: the institutions we alluded to have been accredited by ONA (National Accreditation Organization) either on level 1 or 2, depending on their implementation process.
Send by e-mail:



